Your Location:Home > Products > chemicals for Printing and dyeing > Indigo Blue


CasNo: 482-89-3
MF: C16H10N2O2
Appearance: dark violet powder
|
Chemical properties |
It appears as blue powder, being odorless and slightly soluble in water, ethanol, glycerol and propylene glycol, insoluble in oil. At 25 ℃, the solubility is 1.6% (water), 0.5% (25% ethanol), 0.6% (25% propylene glycol). The 0.05% aqueous solution exhibits dark blue. It has poor performance on all the following aspects: light resistance, heat resistance, acid resistance, alkali resistance, salt tolerance, oxidation resistance and resistance to bacteria. Upon reduction, the color will fade, but the dye is good. The maximum absorption wavelength (610 ± 2) nm. Rat oral LD50: 2g/kg, mice oral LD50 2.5g/kg, ADI 0-5mg/kg (FAO/WHO, 1994). Indigo Aluminum precipitate is a fine powder with violet blue color, being odorless. It can’t dissolve in water and organic solvents with better light resistance and heat resistance than indigo. |
|
Production method |
It is a kind of edible natural blue pigment made from the leaves of Polygonum tinctorium. The indigo leaves are piled up and frequently subject to watering, to ferment 2 to 3 months to become a black clod like. After ramming using mortar, it is known as the ball indigo with the indigo pigment being 2% to 10%. The wood ash, lime and bran are incorporated into ball indigo and further subject to water mixing, heated to 30~40 ℃, exposed to the air to become insoluble blue indigo. Phenylglycine is taken as raw material and form indole phenol after subjecting to alkali melting, followed by air oxidation to derive the products. There are many ways to synthesize phenylglycine. In our country, the condensation method of aniline and chloroacetic acid is adopted. For the convenience of the refining of phenylglycine, we can first make its insoluble iron salt to remove the impurities before converting into soluble sodium salt and entering into alkali melting process. . (1) indigo preparation. Edible indigo is actually indigo disulfonic acid disodium. Indigo is subject to sulfonation with concentrated sulfuric acid, followed by dilution with and then soda ash neutralization. Finally add sodium chloride for salting out, filter, wash and dry to get the finished product. (2) Preparation of indigo aluminum precipitate. First have aluminum chloride and aluminum sulfate have reaction with alkali such as sodium carbonate for preparation of aluminum hydroxide. Then add it to the indigo water solution for precipitation to derive the products. |
|
Hazards & Safety Information |
Category : Toxic substances Toxic classification :? poisoning Acute toxicity :? Oral-mouse LD50> 32000 mg/kg; celiac-mouse LD50: 2200 mg/kg Flammability and Hazardous properties :? being Combustible with combustion producing toxic nitrogen oxide fumes Storage and transportation characteristics:? Ventilated, low temperature and drying Fire extinguishing agent :? dry powder, foam, sand, carbon dioxide, mist water |
|
History |
Indigotin. The blue dye of the ancient world was derived from indigo and woad. Which plant is the oldest is a matter of conjecture. That indigo was known at least four thousand years ago is evident from ancient Sanskrit writings. Cloth dyed with indigotin (CI Natural Blue; CI 75780) has been found in Egyptian tombs and in the graves of the Incas in South America. Indigo belongs to the legume family. The two most important species are Indigo tinctoria and I. suffruticosa, found in India and the Americas, respectively. The leaves of the indigo plant do not contain the dye as such, but in the form of its precursor, a glycoside known as indican. |
|
Definition |
A double indole derivative. |
|
Production Methods |
The first synthesis of indigo is attributed to Adolf von Baeyer (1835–1917), who began hisquest to synthesize indigo in 1865 but was not able to produce indigo until 1878. The syntheticproduction of indigo was first described by Baeyer and Viggo Drewson in 1882; Baeyeralso identified the structure of indigo in 1882.the Baeyer-Drewson synthesis of indigo startedwith 2-nitrobenzaldehyde and acetone proceeding through a series of steps in alkali solution.Baeyer’s work was not commercially viable, and it was not until 1897 that BASF (BadischeAnalin und Soda Fabrik) started to produce indigo commercially using a process developedby Karl von Heumann (1851–1894) that started with naphthalene. The synthetic productionof indigo spelled the end of traditional methods of indigo production. By the second decadeof the 20th century, nearly all indigo was produced synthetically. |
|
General Description |
Dark blue powder with coppery luster. Occurs in isomeric forms (cis and trans). In solid state Indigo is in the trans form. |
|
Air & Water Reactions |
Insoluble in water. |
|
Health Hazard |
ACUTE/CHRONIC HAZARDS: Indigo may cause irritation of the skin and mucous membranes. |
|
Fire Hazard |
Flash point data for Indigo are not available but Indigo is probably combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx. |
|
Properties and Applications |
TEST ITEMS SPECIFICATION APPEARANCE BLUE POWDER SHADE GREENISH HEAT RESISTANCE 180 °C min LIGHT FASTNESS 7 ACID RESISTANCE 3 ALKALI RESISTANCE 4 FASTNESS TO BLEEDING 4 OIL ABSORPTION 40-50% SPECIFIC SURFACE 27 m 2 /g DENSITY 1.60 g/cm 3 RESIDUE ON 80 MESH 5.0% max WATER SOLUBLE 1.0% max VOLATITE 105 °C 1.0% max TINTING STRENGTH 100-105 % |
|
TEST ITEMS |
SPECIFICATION |
|
APPEARANCE |
BLUE POWDER |
|
SHADE |
GREENISH |
|
HEAT RESISTANCE |
180 °C min |
|
LIGHT FASTNESS |
7 |
|
ALKALI RESISTANCE |
4 |
|
FASTNESS TO BLEEDING |
4 |
|
OIL ABSORPTION |
40-50% |
|
SPECIFIC SURFACE |
27 m 2 /g |
|
DENSITY |
1.60 g/cm 3 |
|
RESIDUE ON 80 MESH |
5.0% max |
|
WATER SOLUBLE |
1.0% max |
|
VOLATITE 105 °C |
1.0% max |
|
TINTING STRENGTH |
100-105 % |
|
Purification Methods |
First reduce indigo in alkaline solution with sodium hydrosulfite, and filter. The filtrate is then oxidised by air, and the resulting precipitate is filtered off, dried at 65-70o, ground to a fine powder, and extracted with CHCl3 in a Soxhlet extractor. Evaporation of the CHCl3 extract gives the purified dye. [Brode et al. J Am Chem Soc 76 1034 1954; spectral characteristics are listed, Beilstein 24 II 233, 24 III/IV 1791.] |
InChI:InChI=1/C16H10N2O2/c19-15-9-5-1-3-7-11(9)17-13(15)14-16(20)10-6-2-4-8-12(10)18-14/h1-8,17-18H
Compounds comprising a cross-linking moi...
Compounds of Formula IA, IB, II, III, IV...
Unspecific peroxygenases (UPOs) are glyc...
An ammonia detection material and a dete...
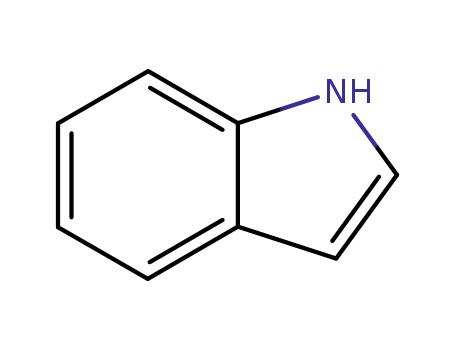
indole

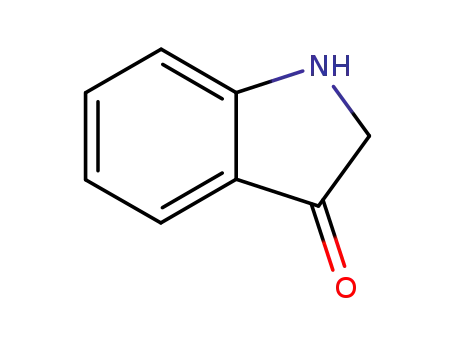
indolin-3-one

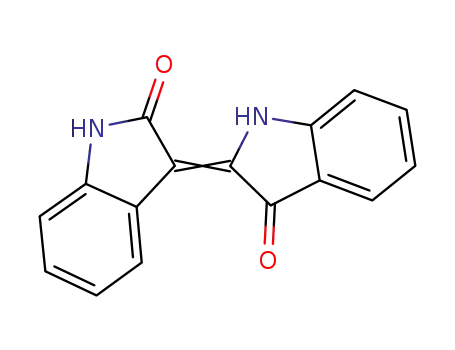
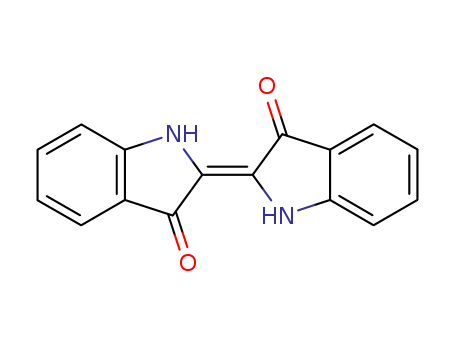
indirubin

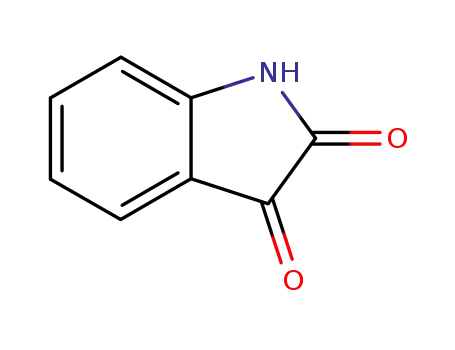
indole-2,3-dione

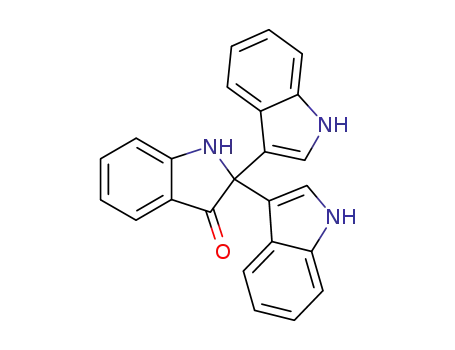
2,2-di(3-indolyl)-3-indolone

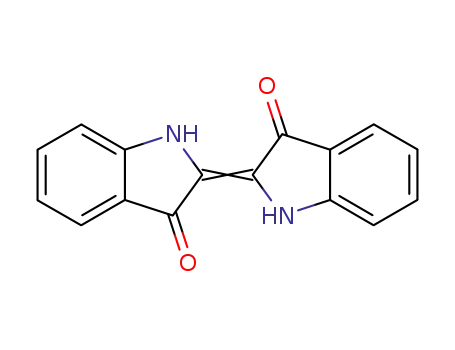
indigo
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide; 3-chloro-benzenecarboperoxoic acid;
In
ethanol;
at 20 ℃;
for 0.333333h;
|
20 %Spectr. 20 %Spectr. 10 %Spectr. 7 %Spectr. 9 %Spectr. |

indole


indolin-3-one

C16H12N2O4


indole-2,3-dione


2,2-di(3-indolyl)-3-indolone


indigo
| Conditions | Yield |
|---|---|
|
With
chloro[5,10,15,20-tetrakis(4-dimethylamino-2,3,5,6-tetrafluorophenyl)porphyrinate]iron(III); dihydrogen peroxide;
In
ethanol;
at 20 ℃;
for 0.333333h;
|
31 %Spectr. 18 %Spectr. 6 %Spectr. 16 %Spectr. 20 %Spectr. |

indole
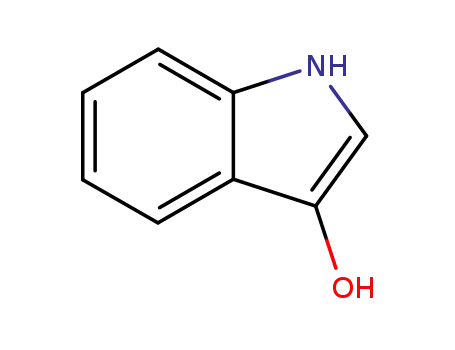
indoxyl
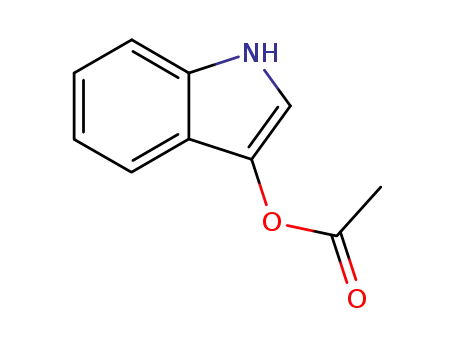
3-indoxyl acetate
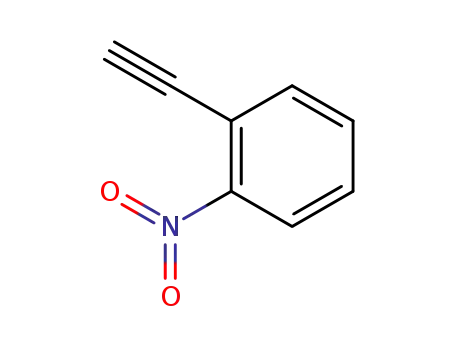
2-ethynyl-1-nitrobenzene
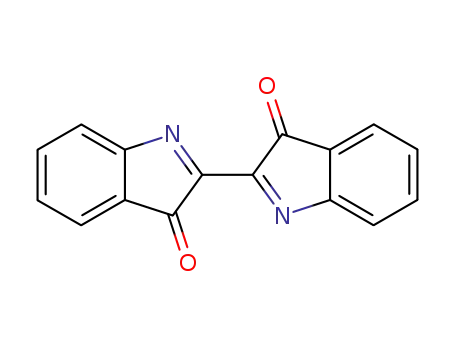
dehydroindigo
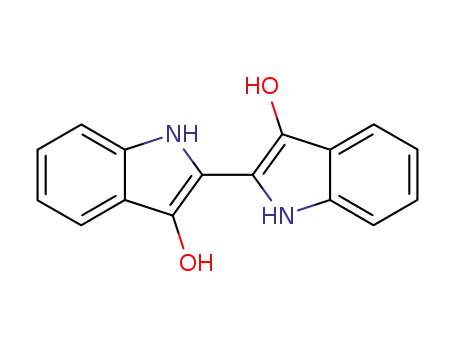
leucoindigo
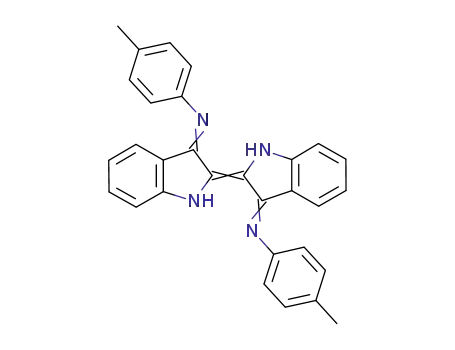
N,N-([2,2'-biindolinylidene]-3,3'-diylidene)bis(4-methylaniline)
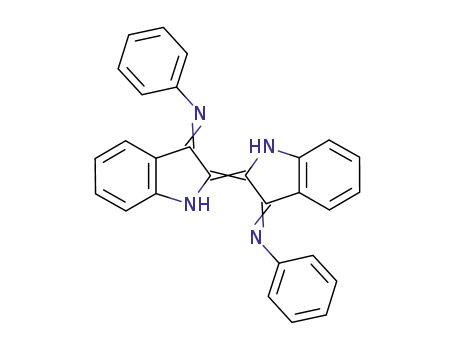
indigo-N,N′-diphenyldiimine