Your Location:Home > Products > chemicals for Water treatment > Cyanuric acid
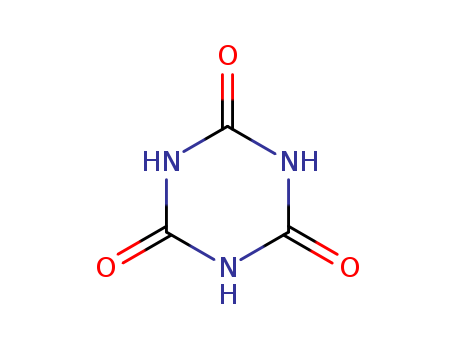


CasNo: 108-80-5
MF: C3H3N3O3
Appearance: white powder
|
Methods of production |
It is obtained by the polymerization of urea. Mixed urea and ammonium chloride, heating and melting, stirring and temperature to 210℃, solution thickened, warming up to 230℃, melting gradually solidified, stir fry evenly, continue to heat up to 250℃, thermal insulation for 15 min, cold to 100℃, adding a small amount of water immersion and down to room temperature in water soaking crushed, filtered solids. The water and hydrochloric acid are added into the solid, stirring and heating to 110 ℃, insulation for 3 h, supplementing with hydrochloric acid and water, cooling to 30 ℃, and washing to neutral, filter, filter cake with water washing and drying to obtain the product. The product purity is ≥95%, consumption of urea 1200kg per ton of product. |
|
Production Methods |
Cyanuric acid is an odorless, crystalline powder. Chlorinated isocyanuratesareusuallypreparedbycontrolledchlorinationof the sodium or potassium salts of cyanuric acid. Other monomeric isocyanurates made from the parent compound include tris(2-hydroxyethyl)isocyanurate and triallyl cyanurate. |
|
Air & Water Reactions |
Soluble in hot water [Hawley]. |
|
Reactivity Profile |
An amide and amine. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generate mixed oxides of nitrogen (NOx) |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
It crystallises from water. Dry it at room temperature in a desiccator in a vacuum. [Beilstein 26 III/IV 632.] |
|
Definition |
cyanuric acid: A white crystallinewater-soluble trimer of cyanic acid,(HNCO)3. It is a cyclic compound havinga six-membered ring made of alternatingimide (NH) and carbonyl(CO) groups (i.e. three -NH-C(O)-units). It can also exist in a phenolicform (three -N=C(OH)- units). |
|
General Description |
Crystals. |
InChI:InChI=1/C3H3N3O3/c7-1-2(8)4-6-5-3(1)9/h(H,6,7)(H2,4,5,8,9)
-
Direct water splitting over photocatalys...
The photoinduced transformation of 2,4,6...
Every year over 250 million pounds of cy...
A study is made of the kinetics and mech...
-
-
-
The aim of this study was to immobilize ...
-
Tripropargylic esters 2 and 10 of cyanur...
1,3,5-Triazine derivatives were screened...
-
Herein, we report the efficient synthesi...
-
General laws of the photochemical oxidat...
A general method was developed for study...
-
-
VUV-irradiation experiments with aqueous...
-
-
The focus of this paper was to identify ...
Phosphorus-doped hexagonal tubular carbo...
Two unexpected one-dimensional coordinat...
-
A new procedure for preparing high-purit...
-
The photocatalytic degradation of atrazi...
-
-
-
-
Selenols are readily oxidized to diselen...
The coupling of graphite (support) with ...
Coordination polymers (CPs) with infinit...
-
The invention discloses a simple, scalab...
bromine

urea

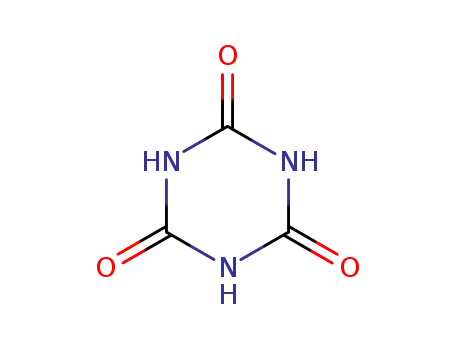
cyanuric acid

ammonium bromide


nitrogen

hydrogen bromide
| Conditions | Yield |
|---|---|
|
|
hydrogenchloride

2,4,6-tris(4-nitrophenoxy)-1,3,5-triazine

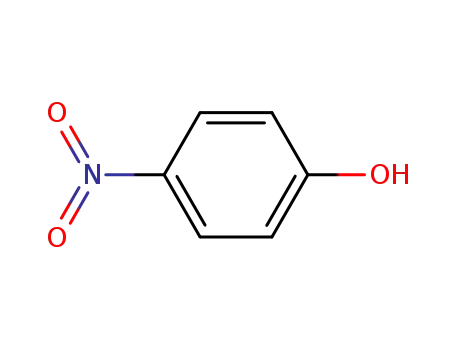
4-nitro-phenol

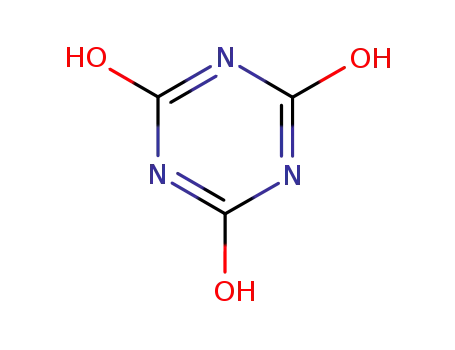
isocyanuric acid
| Conditions | Yield |
|---|---|
|
|
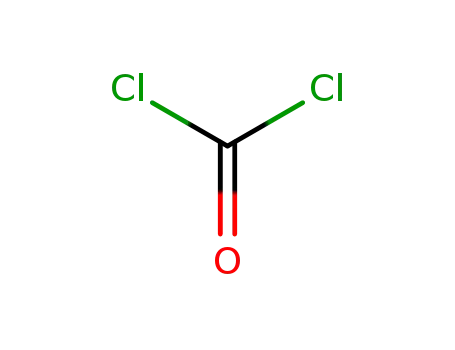
phosgene
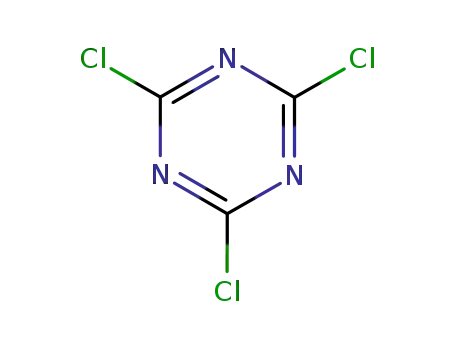
1,3,5-trichloro-2,4,6-triazine
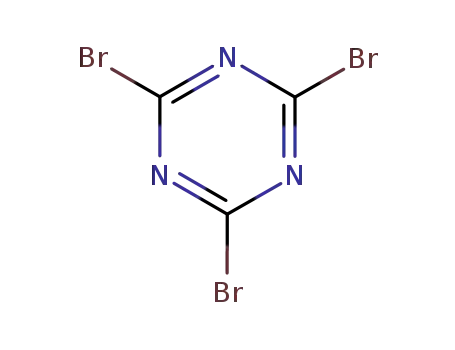
cyanuric bromide
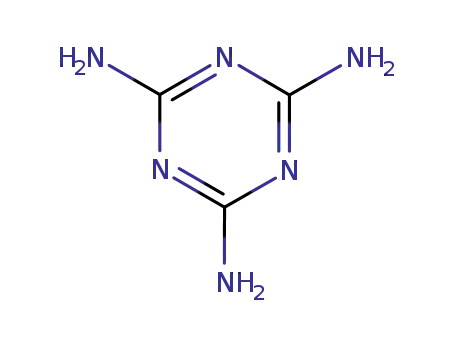
2,4,6-triamino-s-triazine
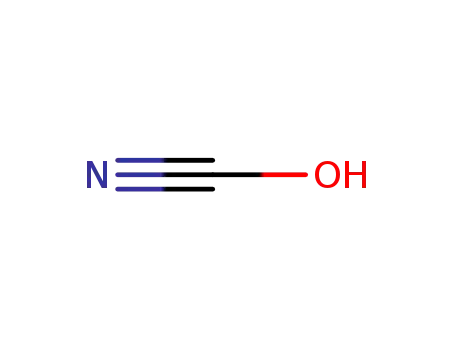
cyanic acid
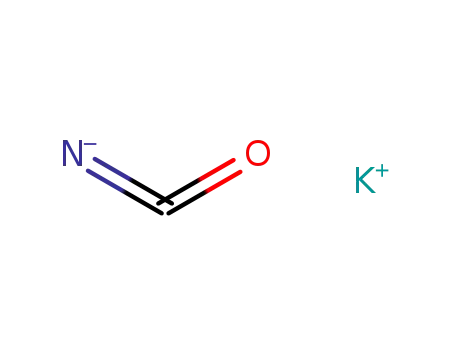
potassium cyanate
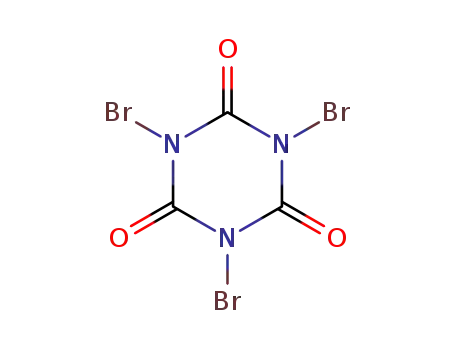
1,3,5-tribromo-1,3,5-triazinane-2,4,6-trione
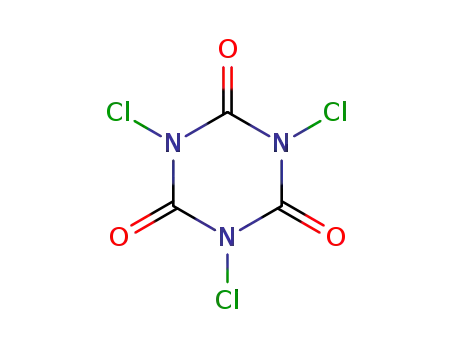
trichloroisocyanuric acid