Your Location:Home > Products > chemicals for Coating > Sodium formate



CasNo: 141-53-7
MF: CHNaO2
Appearance: white crystals
|
SEI Formation and Sodium Dendrite Suppression |
Sodium formate (HCOONa) is a component of the solid electrolyte interphase (SEI) formed naturally on sodium metal anodes. It shows promise in designing artificial SEI layers to suppress sodium dendrite formation and reduce side reactions between sodium and the electrolyte, thus enhancing battery performance. |
|
Hydrogen Production from Formic Acid |
Sodium formate (SF) has long been used as a technological additive for releasing hydrogen (H2) in the dehydrogenation of formic acid. Direct hydrogen production by decomposing SF is of significant importance, especially considering the practical applications in the synthesis of formic acid. |
|
Biogas Production and System Stability |
In the context of biogas production from organic wastes via anaerobic digestion (AD), sodium formate plays a beneficial role in acetogenesis during propionate degradation, contributing to system stability. Its biologically non-toxic nature and neutral pH make it suitable for such applications without acidifying the fermentation system. |
|
Drilling and Completion Fluids |
Sodium formate is utilized in the preparation of brine solutions for drilling and completion fluids, particularly in challenging environments like the Barents Sea. These fluids, made from formate brine solutions and water-soluble polymers, exhibit low viscosity and are colorless, making them suitable for drilling without causing damage to formations. |
|
Chemical Reduction and Bleaching |
Sodium formate chemically reduces other components by donating electrons, making it useful in various chemical processes. It is used in the manufacture of sodium hydrosulfite, a reductive bleaching chemical, and contributes to improving brightness and color in printing fabrics and paper. |
|
Challenges in Powder Handling |
The use of sodium formate powder in industrial processes faces challenges such as particle agglomeration and fusion, which can lead to difficulties in storage and handling. These issues need to be addressed to ensure efficient utilization of sodium formate in various applications, particularly in brine preparation. |
|
Chemical properties |
It is a white powder, with water absorption and a slight odor of formic acid. It is dissolved in water and glycerol, slightly soluble in ethanol and insoluble in ether. |
|
Solubility in water |
Dissolved quantity per 100 ml of water at different temperatures (℃) in grams: 43.9g/0 ℃, 62.5g/10 ℃, 81.2g/20 ℃, 102g/30 ℃, 108g/40 ℃ 122g/60 ℃, 138g/80 ℃, 147g/90 ℃, 160g/100 ℃ |
|
Sodium formate and calcium formate |
Sodium formate and calcium formate are two kinds of common metal salts of formic acid, sodium formate, also known as sodium formate, There are two kinds of molecular forms of sodium formate compounds in nature: ① as a white crystalline powder, anhydrous sodium formate is slightly hygroscopic, poisonous. The relative density is 1.92 (20 ℃), m.p. is 253 ℃. It is dissolved in water, slightly soluble in ethanol and insoluble in ether. ② sodium formate dihydrate is colorless crystals. With slight formic acid odor, it is toxic. It is dissolved in water and glycerine, slightly soluble in ethanol. It broke down into hydrogen and sodium oxalate under strong heat, and finally converted into sodium carbonate. It is produced by the interaction of formic acid and sodium hydroxide. The main purpose of sodium formate as follows: Sodium formate can be used as chemical analysis reagent, used for determination of arsenic and phosphorus, also used as a disinfectant, mordant and so on. Powdered sodium formate is used in industrial instand of formic acid to improve the performance of limestone FGD systems, the advantage is application safety and health. Preparation of sodium formate: Sodium bicarbonate reacts with formic acid in laboratory, remain the solution basic, remove Fe3 +, filtered, and add formic acid into the filtrate, and the solution was acidic, then evaporate, crystallize to obtain crude sodium. Calcium is a free-flowing white crystalline powder, with mouldproof, fungicidal, antibacterial effects, is an organic acid feed additive. Its content is 99%, with 69% formic acid, 31% calcium, low water content. This product has a high melting point, is not easy to be destroyed in the particle mass. Feed is added 0.9% to 1.5%. This product is broken down into formic acid in the stomach, reduces the pH of the stomach, maintains digestive acidity, prevents the growth of bacteria to control and prevent bacterial infection-related diarrhea. Trace acid activates pepsinogen role in enhancing the absorption of active ingredients in feed, and producing chelation with minerals in feed to promote digestion and absorption of minerals, also be used as a supplement of calcium. For the prevention of diarrhea of piglets and improve survival, promote feed conversion and daily gain. The above information is collected and finished by Xiaonan of lookchem. |
|
Production method |
1. It is obtained in industrial by the reaction of carbon monoxide with sodium hydroxide at 150-170 ℃, about 2MPa. In fact, the production process of sodium formate is part of the production of sodium oxalate, the concentration of sodium hydroxide solution for absorbing the reaction was 25%-30%. Sodium formate can be produced by the reaction of formic acid with oxygen or sodium bicarbonate. Material consumption fixed: Carbon monoxide (> 28%) 1630kg/t, caustic soda (> 30%) 2160kg/t. 2. It is the byproduct of pentaerythritol. 2. It is obtained by the reaction of carbon monoxide with sodium hydroxide at 160 ℃, 2MPa. |
|
Physical properties |
White crystals; slightly hygroscopic; faint odor of formic acid; density 1.92 g/cm3; melts at 253°C; decomposes on further heating, first forming sodium oxalate and hydrogen and then sodium carbonate; very soluble in water; the aqueous solution neutral, pH about 7; soluble in glycerol; slightly soluble in alcohol; insoluble in ether. |
|
Definition |
ChEBI: An organic sodium salt which is the monosodium salt of formic acid. |
|
Preparation |
Sodium formate can be prepared in the laboratory by neutralizing formic acid with sodium carbonate. It can also be obtained by reacting chloroform with an alcoholic solution of sodium hydroxide. CHCl3 + 4NaOH → HCOONa + 3NaCl + 2H2O or by reacting sodium hydroxide with chloral hydrate. C2HCl3(OH)2 + NaOH → CHCl3 + HCOONa + H2O The latter method is, in general, preferred to the former because the low aqueous solubility of CHCl3 makes it easier to separate out from the sodium formate solution, by fractional crystallization, than the soluble NaCl would be. For commercial use, sodium formate is produced by absorbing carbon monoxide under pressure in solid sodium hydroxide at 160 °C. CO + NaOH → HCOONa Sodium formate may also be created via the haloform reaction between ethanol and sodium hypochlorite in the presence of a base. |
|
General Description |
Sodium formate is the colorless sodium salt of formic acid. It can be prepared by reacting formic acid with sodium hydroxide or carbonate. Its crystal structure has been investigated. Its crystals exhibit monoclinic-holohedral symmetry. |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Moderately toxic by ingestion, intravenous, and subcutaneous routes. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of NazO. See also FORMIC ACID. |
|
Purification Methods |
A saturated aqueous solution at 90o (0.8mL water/g) is filtered and allowed to cool slowly. (The final temperature should be above 30o to prevent formation of the hydrate.) After two such crystallissations, the crystals are dried in an oven at 130o, then under high vacuum. [Westrum et al. J Phys Chem 64 1553 1960, Roecker & Meyer J Am Chem Soc 108 4066 1986.] The salt has also been recrystallised twice from 1mM DTPA (diethylenetriaminepentaacetic acid, which was recrystallised 4x from MilliQ water and dried in a vacuum), then twice from water [Bielski & Thomas J Am Chem Soc 109 7761 1987]. [Beilstein 2 IV 3.] |
InChI:InChI=1/CH2O2.Na/c2-1-3;/h1H,(H,2,3);/q;+1/p-1
A novel pincer ligand based on the pyraz...
The catalytic hydrogenation of carbon di...
We discovered using SECM of the electro-...
The novel title polymeric copper(II) com...
Copper metal and copper-based amorphous ...
CO2 hydrogenation to formic acid/formate...
Direct reduction of bicarbonate, a typic...
The hydrogenation of carbon dioxide into...
Herein, we report a novel amino acid bas...
-
Borohydrides are widely used reducing ag...
Nanoporous Ag-Sn was prepared by direct ...
Increasing atmospheric CO2 levels have g...
The hepta-coordinated isomeric M(NO)Cl3(...
A highly efficient recyclable system for...
Molecular catalyst-based direct hydrogen...
Fe(II) hydrido carbonyl complexes suppor...
-
Well-defined ruthenium(ii) PN3P pincer c...
The electrodes of Cu and Cu-based amorph...
[RuCl(L1)(MeCN)2]Cl (1) and [RuCl(L2)(Me...
Formic acid, isolable as sodium formate ...
In a previous work from our laboratory, ...
Kinetic data on elelctroless copper depo...
Formate salts are important chemicals wi...
Raman spectra of the hydrogen-deuterium ...
Bismuth vanadate is a widely known photo...
Increased carbon dioxide (CO2) emissions...
The kinetics of the autocatalytic reacti...
Formic acid and formate salts are key in...
The invention discloses a preparation me...
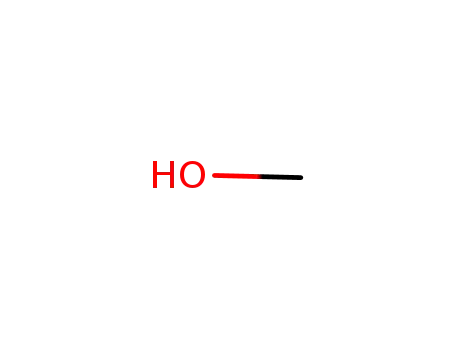
methanol

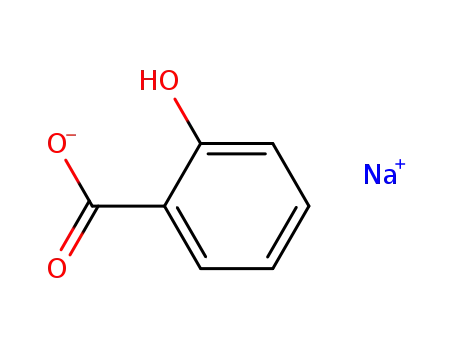
sodium salicylate

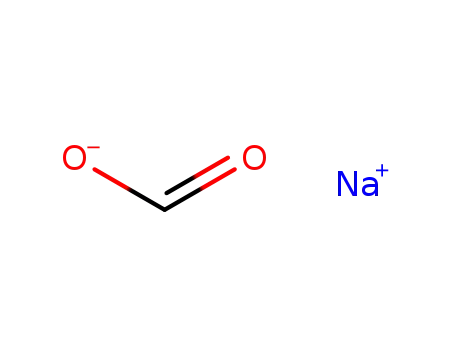
sodium formate

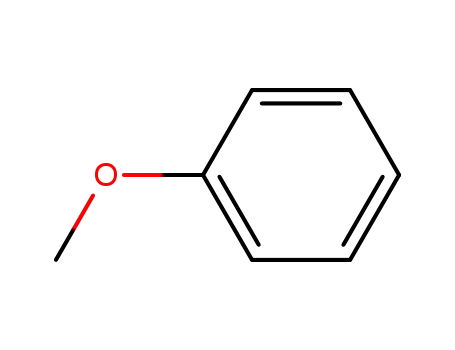
methoxybenzene

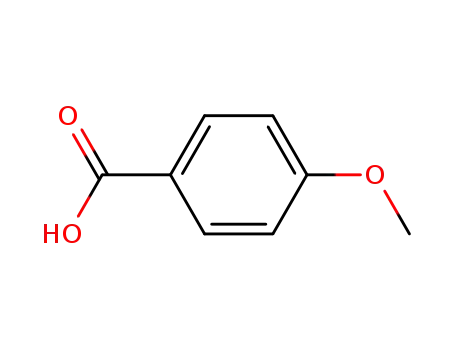
4-methoxybenzoic acid

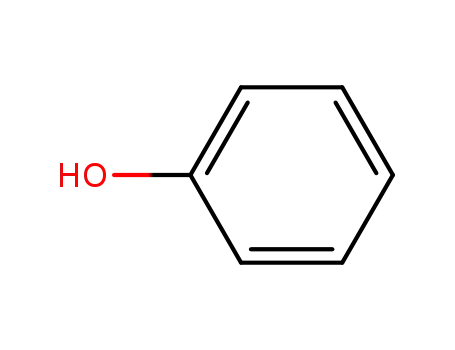
phenol
| Conditions | Yield |
|---|---|
|
at 180 ℃;
|
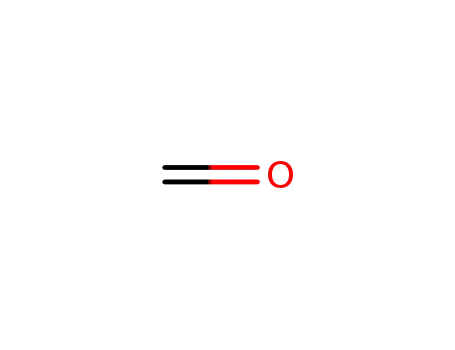
formaldehyd

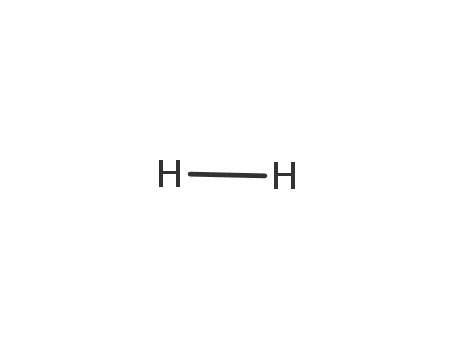
hydrogen


sodium formate
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
tungsten monocarbide;
In
sodium hydroxide;
byproducts: CH3OH; tungsten carbide added to formaldehyde soln. containing NaOH at room temp. under atm. pressure; identified by gas chromy.;
Kinetics;
|
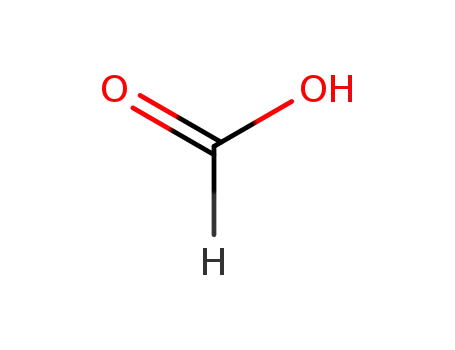
formic acid
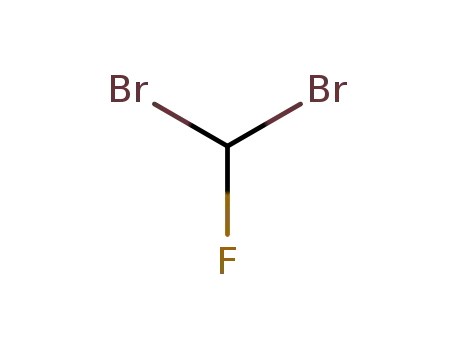
dibromofluoromethane
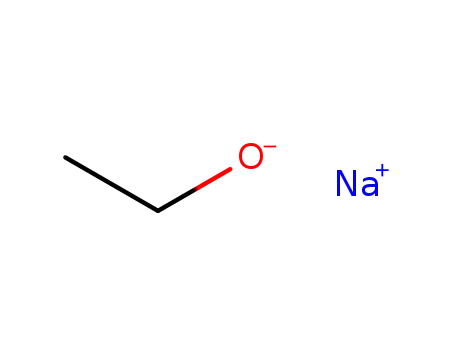
sodium ethanolate
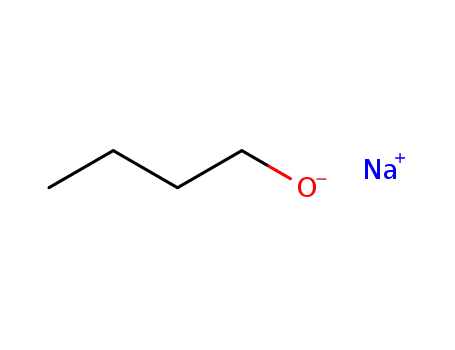
sodium butanolate
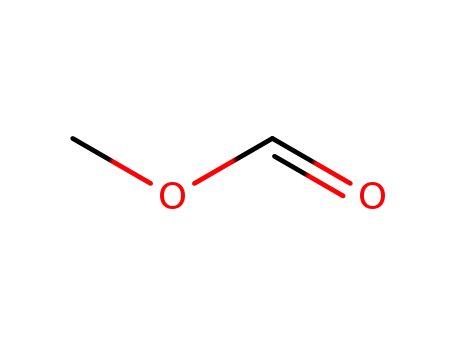
Methyl formate
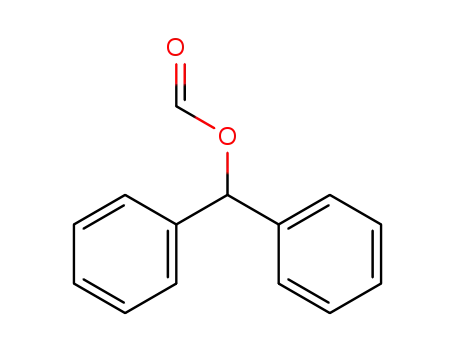
benzhydryl formate
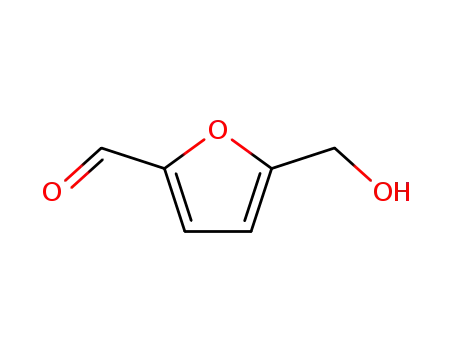
5-hydroxymethyl-2-furfuraldehyde
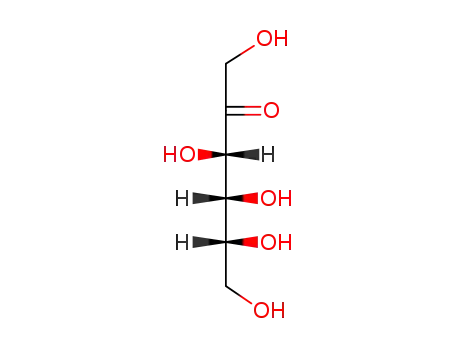
D-Fructose